Background
Tinostamustine, a novel alkylating deacetylase inhibitor, improves drug access to cancer cell DNA strands, breaks them and counteracts damage repair. Tinostamustine was well tolerated, with signals of efficacy, during the dose-escalation stage of a Phase I study in patients with relapsed/refractory (R/R) haematological malignancies for whom no standard treatment with proven clinical benefit was available or recommended.
Aims
Here we report safety and efficacy findings for tinostamustine in an expansion cohort of patients with CTCL from the Phase I study in patients with advanced haematological malignancies.
Methods
Patients with advanced CTCL (either mycosis fungoides [MF] or Sezary syndrome [SS]), for whom no standard treatment with proven clinical benefit was available or recommended, received the recommended Phase II dose (RP2D) of tinostamustine 80 mg/m 2 (2 patients) and 100 mg/m 2 (11 patients) over 60 minutes on Day 1 of a 21-day cycle. Patients with CTCL who had received ≥1-4 prior standard systemic therapies were eligible to be enrolled into the cohort. Primary objectives included evaluation of overall response and the safety of tinostamustine. Adverse events (AEs) were assessed for severity using US National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE; version 4.03, June 2010) with the exception that assessment of QTc prolongations was based on CTCAE version 5.0. Treatment-related AEs, AEs leading to death, serious adverse events (SAEs), and AEs resulting in trial discontinuation were recorded. Efficacy objectives estimated the overall response rate (ORR; complete response [CR] + partial response [PR]) and clinical benefit rate (CBR; CR + PR + stable disease ≥4 cycles [SD]) of tinostamustine in patients with CTCL. Secondary efficacy variables included progression-free survival (PFS) and overall survival (OS); all outcomes were evaluated from Cycle 3 until progression or toxicity.
Results
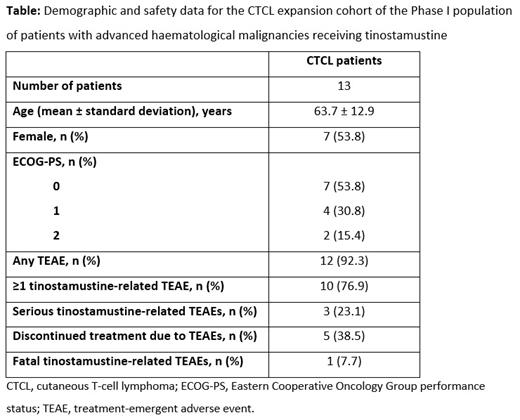
Out of 48 patients with advanced haematological malignancies treated in the expansion-cohort stage of this Phase I study, 13 had CTCL (MF, n=9; SS, n=4; Table). Mean ± standard deviation age was 63.7 ± 12.9 years, 7/13 (53.8%) patients were female and over half of patients had an Eastern Cooperative Oncology Group performance status score of 0. At the end of the study, patients with CTCL had received a median of 5.0 (range 1-8) cycles of tinostamustine. No unexpected AEs were observed following tinostamustine treatment. Treatment-emergent adverse events (TEAEs) occurred in 12/13 (92.3%) patients. Gastrointestinal AEs were observed in 9/13 patients, the majority of which were nausea or vomiting. Haematological AEs, including thrombocytopenia and anaemia, occurred in 8/13 patients. Eleven serious TEAE events that were considered related to tinostamustine occurred in three patients ( Table) and included two events each of thrombocytopenia, neutropenia and leukopenia, and one event each of anaemia, lymphopenia, lichenoid keratosis, acute coronary syndrome, and interstitial lung disease. There were no incidences of QTcF >500 ms or increased >60 ms from baseline. Overall, 3/13 patients (23.1%) discontinued due to progressive disease and 5/13 (38.5%) due to AEs. One patient with MF experienced a fatal TEAE of decreased appetite. 12/13 patients in the safety analysis were evaluated for disease response. 2/12 patients achieved a CR (1 patient with MF, 1 with SS), 4/12 a PR (3 patients with MF, 1 with SS) and 4/12 experienced SD (2 patients with MF, 2 with SS). ORR was 50% (6/12 patients; 95% confidence interval [CI]: 21.1%, 78.9%) and CBR 83.3% (10/12 patients; 95% CI: 51.6%, 97.9%). Median PFS was 7.3 months (95% CI: 3.2, NE months; 6/12 patients progressed) and median OS was not estimable (4/12 patients died).
Conclusions
Initial results from this expansion cohort of patients with CTCL further demonstrate signals of efficacy in this heavily pre-treated patient population for whom no other standard therapy with proven clinical benefit was available or recommended; an ORR of 50%, with 6/12 patients achieving a CR or PR, and a median progression-free survival of 7.3 months were observed. Tinostamustine was well tolerated, with no unexpected AEs. The principal TEAEs were haematological and gastrointestinal.
Funding: Mundipharma Research Limited and Purdue Pharma, LP
Disclosures
Zinzani:EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Morschhauser:F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy; F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees. Janik:Mundipharma Research Ltd: Current Employment. Manamley:Mundipharma Research Ltd: Current Employment. Hilgier:Mundipharma Research Ltd: Consultancy. Sureda Balari:Roche: Honoraria; Sanofi: Consultancy, Honoraria; Gilead: Consultancy; Gilead Kite: Honoraria; Amgen: Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; MSD: Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Alexion: Honoraria.